The relationship between substance use and endocrine health is a complex field of clinical study, often clouded by anecdotal evidence and historical stigmas. Among the various concerns cited by patients and clinicians alike, the question of whether marijuana consumption leads to the development of “man boobs,” or glandular breast tissue enlargement, remains a primary focus. For decades, researchers have investigated the central query: does cannabis cause gynecomastia? While early case reports suggested a definitive link, contemporary longitudinal studies present a more nuanced and sometimes contradictory perspective on how Delta-9-tetrahydrocannabinol (THC) interacts with the human hormonal milieu.
Does Cannabis Cause Gynecomastia? Clinical Evidence and Hormonal Realities
The Endocrine Mechanisms of THC
To understand the potential for cannabis to influence breast tissue growth, one must examine the hypothalamic-pituitary-gonadal (HPG) axis. This system regulates the production of testosterone and estrogen, the two primary hormones responsible for maintaining secondary sexual characteristics. Gynecomastia occurs when there is an imbalance in the ratio of free estrogen to androgens, specifically an excess of estrogenic activity relative to testosterone levels.
The Role of the HPG Axis
THC, the primary psychoactive component of cannabis, interacts with the endocannabinoid system, which is intrinsically linked to hormonal regulation. Some studies suggest that high doses of THC can inhibit the release of Gonadotropin-Releasing Hormone (GnRH). This inhibition leads to a cascade effect, reducing the secretion of Luteinizing Hormone (LH) and subsequently lowering serum testosterone levels.
THC and Estrogen Mimicry
Beyond testosterone suppression, there is a theoretical concern that certain compounds within the cannabis plant may act as phytoestrogens. These plant-based chemicals can bind to estrogen receptors in human tissue, potentially stimulating the growth of glandular breast tissue. While the estrogenic potency of cannabis is significantly lower than endogenous estradiol, chronic exposure in susceptible individuals may contribute to the morphological changes associated with gynecomastia.
Analyzing the Evidence: Does Cannabis Cause Gynecomastia?
The medical community first flagged this issue in 1972, following a series of case reports involving heavy cannabis users. However, these early observations lacked the control groups necessary to establish a direct causal relationship. In the intervening decades, the scientific consensus has shifted toward a position of “potential but unproven” correlation.
| Study Period | Primary Findings | Scientific Consensus |
|---|---|---|
| 1970s – 1980s | Frequent case reports and small-scale observational data. | High suspicion of direct causality. |
| 1990s – 2010s | Larger epidemiological studies and animal models. | Inconsistent results; weak correlation in humans. |
| 2020s – Present | Advanced hormonal profiling and molecular analysis. | Likely dependent on dosage, genetics, and co-factors. |
Current data suggests that while cannabis use can lead to transient changes in hormone levels, these changes do not always translate into clinical gynecomastia. Individual sensitivity to cannabinoid-induced endocrine shifts appears to be a significant variable, meaning that some users may experience tissue growth while others remain entirely unaffected despite similar consumption patterns.
Comparative Risks and Other Contributing Factors
When assessing a patient for gynecomastia, clinicians must differentiate between cannabis-related effects and other common causes. Often, cannabis use is concurrent with other lifestyle factors that are more strongly linked to hormonal disruption, such as alcohol consumption or a high body mass index (BMI).
- Adiposity: Excess body fat contains the enzyme aromatase, which converts testosterone into estrogen.
- Alcohol Consumption: Ethanol is a known endocrine disruptor that can impair liver function, leading to decreased estrogen clearance.
- Medication Side Effects: Many prescriptions, including certain antidepressants and heart medications, have a higher statistical correlation with gynecomastia than cannabis.
| Substance/Condition | Mechanism of Action | Impact Level |
|---|---|---|
| Anabolic Steroids | Direct aromatization to estrogen | High |
| Chronic Alcoholism | Liver dysfunction and direct toxicity | Moderate to High |
| Cannabis Use | HPG axis suppression (theoretical) | Low to Moderate |
| Obesity | Peripheral aromatization of androgens | High |
Key Takeaways for Patients
For individuals concerned about the physical effects of marijuana use, several insights are critical for managing health outcomes:
- Dosage Matters: Occasional use is unlikely to trigger significant hormonal shifts compared to chronic, high-potency consumption.
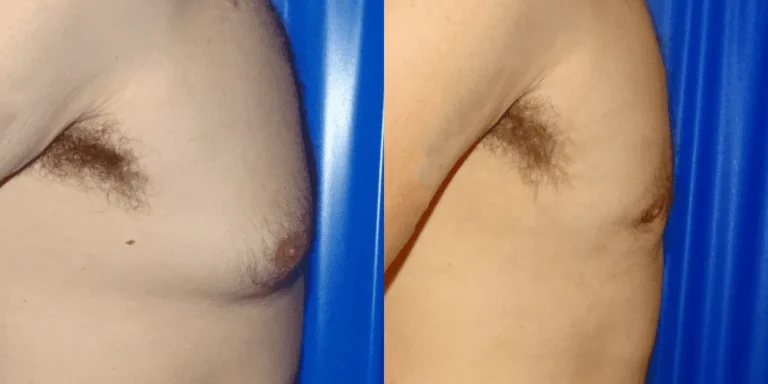
- Reversibility: In many cases, substance-induced gynecomastia can recede if the offending agent is removed during the early “florid” phase.
- Medical Consultation: Sudden changes in breast tissue should always be evaluated by a healthcare professional to rule out underlying pathologies like testicular tumors or hyperprolactinemia.
- Healthy Lifestyle: Mitigating other risk factors, such as weight gain and excessive alcohol intake, can provide a protective effect against hormonal imbalances.
Frequently Asked Questions
Is cannabis-induced gynecomastia permanent?
In the early inflammatory stages, the tissue growth may be reversible if cannabis use is discontinued. However, if the tissue remains for more than 12 to 18 months, it often becomes fibrotic, at which point surgical intervention may be required to remove the glandular material.
Does the method of consumption (smoking vs. edibles) change the risk?
While the pharmacokinetic profile differs between inhalation and ingestion, both methods deliver THC into the bloodstream where it can interact with the HPG axis. There is currently no definitive evidence suggesting that one method is significantly riskier than the other regarding gynecomastia.
Can CBD cause similar hormonal issues?
Cannabidiol (CBD) does not appear to have the same impact on the HPG axis as THC. Most studies focusing on endocrine disruption have identified THC as the primary compound of concern due to its affinity for CB1 and CB2 receptors in the hypothalamus and pituitary gland.